Details of the Drug
General Information of Drug (ID: DMZJYBW)
| Drug Name |
Metocurine Iodide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Metokuriinijodidi; Metokurinjodid; Metubine; Dimethyl tubocurarine iodide; Dimethylchondrocurarine iodide; Dimethyltubocurarine Iodide; Metocurine diiodide; Metocurine iodide [USAN]; Metocurini Iodidum; Metubine iodide; Mutubine Iodide; Trimethyltubocurarine Iodide; Dimethylether of d-tubocurarine iodide; Dimetiltubocurarinio, ioduro de; Methyl-curarin; Methyl-curarin [German]; Metocurine iodide (USAN); Metubine iodide (TN); O,O'-Dimethylchondrocurarine diiodide; Tubocurarine, O,O'-dimethyl-, diiodide; Tubocuraranium, 6,6',7',12'-tetramethoxy-2,2,2',2'-tetramethyl-, diiodide; (+)-O,O'-Dimethylchondrocurarine Di-iodide; (+)-O,O'-Dimethylchondrocurarine diiodide; 2,2,2',2'-tetramethyl-6,6',7',12'-tetrakis(methyloxy)tubocuraran-2,2'-diium diiodide; 6,6',7',12'-Tetramethoxy-2,2,2',2'-tetramethyltubocuraranium diiodide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anesthetics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
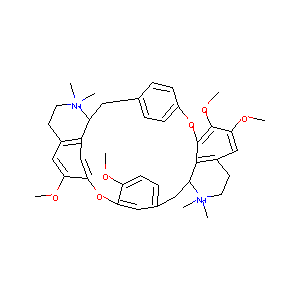 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight | 906.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 8 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Anaesthesia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 9A78.6 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
